|
A fuel cell is an electrochemical energy
conversion device. A fuel cell converts the chemicals hydrogen and oxygen
into water, and in the process it produces electricity.
The other electrochemical device that we are all familiar
with is the battery. A battery has all of its chemicals stored inside, and it
converts those chemicals into electricity too. This means that a battery
eventually "goes dead" and you either throw it away or recharge it.
With a fuel cell, chemicals constantly flow into the cell so
it never goes dead -- as long as there is a flow of chemicals into the cell, the
electricity flows out of the cell. Most fuel cells in use today use hydrogen and
oxygen as the chemicals.
Fuel cells are generally categorized by their
electrolyte�the material sandwiched between the two electrodes. This
material's characteristics determine the optimal operating temperature and the
fuel used to generate electricity.
|
Fuel Cell Type
|
Electrolyte |
Anode
Gas |
Cathode
Gas |
Temperature |
Efficiency |
Proton
Exchange Membrane
(PEM) |
Solid
polymer membrane |
Hydrogen |
Pure
or atmospheric oxygen |
75�C
(180�F) |
35�60% |
Alkaline
(AFC) |
Potassium
hydroxide |
Hydrogen |
Pure
oxygen |
below
80�C |
50�70% |
Direct
Methanol
(DMFC) |
Solid
polymer membrane |
Methanol
solution in water |
Atmospheric
oxygen |
75�C
(180�F) |
35�40% |
Phosphoric
Acid
(PAFC) |
Phosphorous |
Hydrogen |
Atmospheric
oxygen |
210�C
(400�F) |
35�50% |
Molten
Carbonate
(MCFC) |
Alkali-
Carbonates |
Hydrogen,
methane |
Atmospheric
oxygen |
650�C
(1200�F) |
40�55% |
Solid
Oxide
(SOFC) |
Ceramic
Oxide |
Hydrogen,
methane |
Atmospheric
oxygen |
800�1000�C
(1500�1800�F) |
45�60% |
Types of Fuel Cells
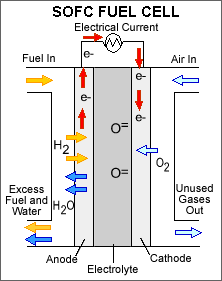
How Solid Oxide fuel Cell Works
|